QT prolongation
The cardiac conduction system (CCS, also called the electrical conduction system of the heart) transmits the signals generated by the sinoatrial node—the heart’s pacemaker, to cause the heart muscle to contract, and pump blood through the body’s circulatory system.
- PQ-interval: Time it takes before the signal has passed the atrioventricular (AV) node.
- QRS-complex: depolarization of the chambers.
- ST-segment and T-wave: repolarization of the chambers (return to “normal”).
Causes of QT-prolongation
Drug inhibition of ion-channels in the heart
Genetics (hereditary)
Hypocalcemia, hypomagnesia
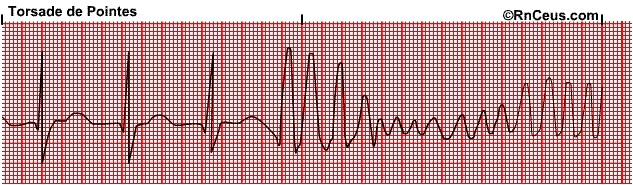
Can in rare cases lead to fainting or death through torsades de pointes (TdP), french for “twisting of peaks”.
QT-prolongation ≠ risk for TdP
There are more factors needed for TdP, e.g. “early afterdepolarizations”.
- QT-time is affected by heat rate: Corrected QTc-time (Bazetts and Fridericia’s formulas)
Thorough QT/QTc (TQT) study
- According to ICH E14 (2005), in general, all drugs shall perform a thorough QT/QTc study early in development.
- Large targeted proteins and monoclonal antibodies have a low likelihood of direct ion channel interactions and a thorough QT/QTc study is generally not necessary. (QA 2015)
- The threshold level of regulatory concern, is around 5 ms as evidenced by an upper bound of the 95% confidence interval around the mean effect on QTc of 10 ms
- Drugs that prolong the mean QT/QTc interval by >20 ms have a substantially increased likelihood of being proarrhythmic
How to perform the study
- The study is typically carried out in healthy volunteers
- Well controlled; positive control group recommended (assay sensitivity)
- Crossover or parallel group study designs
- Timing of the collection of ECGs and the study design (e.g., single or multiple dose, duration) should be guided by PK. For drugs with short half-lives and no metabolites, a single dose study might be sufficient.
- Studies should characterize the effect of a drug on the QT/QTc throughout the dosing interval.
- Dose-response and generally the concentration-response for QT/QTc prolongation should be characterized
- The drug should be tested at substantial multiples of the anticipated maximum therapeutic exposure, unless there are other safety/tolerability concerns.
- The QT/QTc interval data should be presented both as analyses of central tendency (e.g., means, medians) and categorical analyses.
Concentration-QTc (C-QTc) study
A well-designed and conducted QTc assessment based on C-QTc modeling in early phase 1 studies can be an alternative approach to a thorough QT study for some drugs to reliably exclude clinically relevant QTc effects.
Aim is to assess drug effect on heart repolarization (same as a TQT-study). A C-QT study can be samller than a TQT-study, since all data from all doses can be used. Does not need to be a separate dedicated study. Can measure e.g. ECG during Phase 1.
How to perform the study
Usually done early (Phase 1), to answer the question “what intensity of ECG-monitoring is needed in later phases?”
SAD/MAD data are well suited for early assessment of C-QTc.
- Sample size—usually 4–8 persons per dose cohort + 2–4 persons getting placebo
- Placebo makes it possible to rule out small QTc effects, allows detection of circadian rythms in QTc-data
- Baseline ECG
- Positive control is usually missing—demands the study of high exposures
- 2 times maximal therapeutic exposure
- Data from several studies are not recommended where bias can be introduced